- SOM
- Departments and Offices
- SOM Departments
- Pathology
- UMMC Lab Services
- Lab Test List
- Laboratory
- Tests
Tests
Main Content
Tobramycin
| Test Name: | Tobramycin | ||
| Epic Order Code: | Peak: | LAB36 | |
| Random: | LAB37 | ||
| Trough: | LAB38 | ||
| CPT Code: | 80200 | ||
| Specimen(s) Type: | Serum | ||
| Acceptable Container(s): | 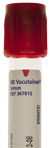 Tube top is red. Tube top is red. | ||
| Testing Schedule: | 24 hrs/day, 7 days/week | ||
| Turn Around Time: | STAT: 1 hour | ||
| Routine: 2 hours | |||
| Collection Information: | No special patient preparation is required. Obtain specimen by standard collection procedures. | ||
| Transport Information: | Deliver to lab immediately. All specimens must be signed into the laboratory. | ||
| Reference Clients: | If unable to deliver to the lab within 1 hour, centrifuge the specimen and remove serum from the red cells. Store and transport at 2-8° C. Specimen must be received in the lab within 24 hours. | ||
| Causes for Rejection: | Improperly labeled, incorrect container, contaminated, insufficient quantity, incorrect/delay in transport. | ||
| Reference Range: | Peak: 6.0-10.0 µg/mL | ||
| Trough: 0.5-2.0 µg/mL | |||
| Critical, Peak & Random: >12.0 µg/mL | |||
| Critical, Trough: >2.1 µg/mL | |||
| Additional Information: | Amikacin cross-reacts with this assay. Kanamycin cross-reacts significantly; however, the assay has not been optimized to quantitate this aminoglycoside. Aminoglycosides are not generally coadministered in clinical practice, although more than one aminoglycoside may be present when switching from treatment with one to another. Samples that contain tobramycin in combination with either amikacin or kanamycin cannot be reliably quantitated by this assay.Endogenous substances such as DLIF (Digoxin-like immunoreactive factors) may interfere with this assay by yielding slightly elevated results. DLIF are observed primarily in samples from neonates, pregnant women, and acute care patients with renal or hepatic failure. As with many mouse monoclonal antibody-based immunoassays, this assay may experience interference with samples containing human anti-mouse antibodies (HAMA). Samples suspected of containing HAMA (e.g., from patients with history of mouse monoclonal antibody exposure) should be tested by an alternate method. | ||


